Overview of Zirconia Ceramics
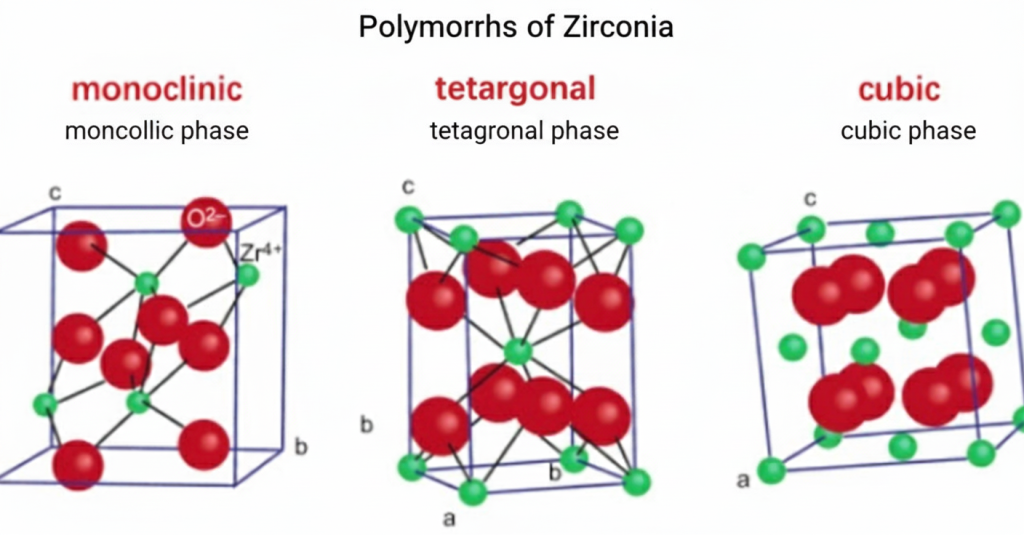
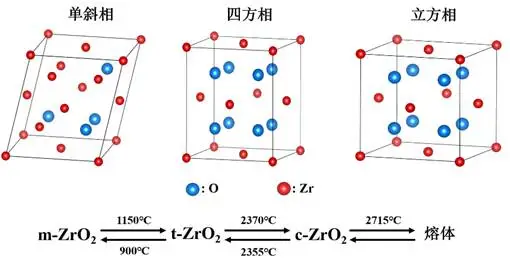
Zirconia is a white crystalline oxide composed of zirconium (Zr) and oxygen (O), with the chemical formula ZrO₂. Pure zirconia exists in three crystalline phases at different temperatures: monoclinic (m-ZrO₂), tetragonal (t-ZrO₂), and cubic (c-ZrO₂). The transformation from the tetragonal phase to the monoclinic phase is accompanied by a volume expansion of approximately 3-5%, which can lead to cracking in ceramics during cooling. To overcome this problem, stabilizers (such as yttrium oxide Y₂O₃, magnesium oxide MgO, calcium oxide CaO, etc.) are typically added to stabilize the high-temperature crystalline phases, thereby achieving a “phase transformation toughening” mechanism and significantly improving the fracture toughness of the material.
زركونيا (زرو₂) ceramics, due to their excellent mechanical properties and unique physicochemical characteristics, occupy a crucial position in the field of advanced ceramics. Known as “ceramic steel,” it is widely used in high-tech fields such as aerospace, biomedicine, electronics and communications, and precision machinery, making it one of the indispensable key materials in modern industry.

Crystalline forms of aluminum oxide
أكسيد الألومنيوم (آل₂O₃) is known to exist in more than ten crystalline structures, including α, γ, β, η, δ, θ, χ, κ-Al₂O₃ and an amorphous phase. Among these, the most common and important are α-Al₂O₃, γ-Al₂O₃, و β-Al₂O₃. These different forms of aluminum oxide exhibit significant differences in structure and properties, which determine their respective application areas.
- Crystal Structure: Belongs to the P2₁/c space group, exhibiting relatively low crystal structure symmetry and a relatively loose atomic arrangement. The unit cell has monoclinic symmetry.
- Stability Conditions: Under normal temperature and pressure, zirconium dioxide exists in the monoclinic phase, which is the stable phase of zirconium dioxide at room temperature.
- Physical Properties: Density is approximately 5.65-5.68 g/cm³, and it has relatively low hardness. The crystal structure is prone to deformation under stress.
- Crystal Structure: It belongs to the P4₂/nmc space group, with a tetragonal unit cell and a more regular and ordered atomic arrangement, exhibiting high crystal symmetry.
- Stability Conditions: When the temperature rises to approximately 1150℃, monoclinic zirconia undergoes a phase transition, transforming into the tetragonal phase. The tetragonal phase is stable in the temperature range of 1150℃-2370℃.
- Physical Properties: The density is approximately 6.10 g/cm³, and the hardness and strength are improved compared to the monoclinic phase. The crystal structure exhibits good stability at high temperatures.
- Crystal Structure: It belongs to the Fm3m space group, with a perfect cubic unit cell and a highly symmetrical arrangement of atoms, making it the most symmetrical phase among zirconium dioxide crystal structures.
- Stability Conditions: When the temperature rises to 2370℃, the tetragonal phase of zirconium dioxide further transforms into the cubic phase. The cubic phase is stable in the temperature range of 2370℃-2715℃ (melting point).
- Physical Properties: It has a density of approximately 6.27 g/cm³, possesses high hardness, strength, and thermal stability, and its crystal structure exhibits excellent performance at high temperatures.
These three crystalline forms of zirconia transform into one another under different temperatures and chemical environments, and their properties and applications vary depending on the crystal structure. By controlling temperature, adding stabilizers, and other methods, the crystal structure of zirconia can be adjusted to meet the application needs of different fields.
Core Performance and Advantages
|
Property
|
Typical Value
|
Unit
|
Remarks
|
|
كثافة
|
5.85 – 6.05
|
g/cm³
|
High density
|
|
Vickers Hardness
|
1200 – 1400
|
الجهد العالي
|
Excellent wear resistance
|
|
Flexural Strength
|
900 – 1200
|
MPa
|
High load-bearing capacity
|
|
Fracture Toughness
|
8 – 12
|
MPa·m^1/2
|
Key characteristic of “ceramic steel”
|
|
Elastic Modulus
|
200 – 220
|
المعدل التراكمي
|
Good rigidity
|
Beyond mechanical properties, zirconia ceramics also exhibit remarkable physicochemical stability:
- High Temperature Resistance: Melting point up to 2715℃, stable operation in high-temperature environments.
- Corrosion Resistance: Strong resistance to chemical media such as acids, alkalis, and salts, suitable for harsh chemical environments.
- Low Thermal Conductivity: Thermal conductivity as low as 2-3 W/(m·K), making it an excellent thermal insulator.
- Thermal Expansion Coefficient: Approximately 10.5 × 10⁻⁶/K, close to metallic materials (على سبيل المثال, steel), facilitating composite bonding with metals.
- Biocompatibility: Non-toxic, non-irritating, and good compatibility with human tissues, making it an ideal biomedical material.
Zirconia Ceramics Properties Table
|
Property |
Unit |
Z-100 |
Z-200 |
Z-300 |
ZM-100 |
ZB-100 |
|---|---|---|---|---|---|---|
|
Material Composition |
– |
ZrO₂-Y₂O₃ |
ZrO₂-Y₂O₃ |
ZrO₂-Y₂O₃ |
ZrO₂-Y₂O₃ |
ZrO₂-Y₂O₃ |
|
Color |
– |
White |
White |
White |
Yellow |
Blue |
|
كثافة |
g/cm³ |
6.00 |
6.03 |
6.04 |
5.70 |
6.03 |
|
Flexural Strength |
MPa |
900 |
950 |
1100 |
450 |
1100 |
|
Compressive Strength |
MPa |
2100 |
2200 |
2300 |
1600 |
2300 |
|
Elastic Modulus |
المعدل التراكمي |
200 |
210 |
220 |
210 |
220 |
|
Fracture Toughness |
MPa·m¹/² |
9.0 |
– |
9.0 |
5.5 |
7.0 |
|
Poisson’s Ratio |
– |
0.3 |
0.3 |
0.3 |
0.3 |
0.3 |
|
صلابة (HRA) |
HRA |
89 |
90 |
90 |
88 |
90 |
|
Vickers Hardness |
HV1 |
1250 |
1450 |
1450 |
1240 |
1450 |
|
Thermal Expansion Coefficient |
10⁻⁶/K |
10 |
10 |
10 |
– |
10 |
|
الموصلية الحرارية |
W/(m·K) |
3 |
3 |
3 |
3 |
3 |
|
مقاومة الصدمات الحرارية |
ΔT°C |
400 |
400 |
400 |
– |
400 |
|
Max Use Temp (Oxidizing) |
درجة مئوية |
1000 |
1000 |
1000 |
850 |
1000 |
|
Max Use Temp (Reducing/Inert) |
درجة مئوية |
1000 |
1000 |
1000 |
850 |
1000 |
|
Volume Resistivity (20درجة مئوية) |
Ω·cm |
10¹³ |
10¹² |
10¹² |
5×10¹³ |
10¹² |
|
Dielectric Strength |
كيلو فولت / مم |
19 |
15 |
17 |
19 |
17 |
|
Dielectric Constant (1MHz) |
– |
28 |
30 |
30 |
27 |
30 |
|
Dielectric Loss (tanδ) |
– |
2×10⁻³ |
2×10⁻³ |
2×10⁻³ |
2×10⁻³ (1GHz) |
2×10⁻³ |
How are zirconia ceramics manufactured?

- Zirconia Raw Material Acquisition: Zirconia is typically extracted from zircon (ZrSiO₄) using chemical or electromelting methods.
- Chemical Method: Zircon is reacted with alkaline substances such as sodium hydroxide to produce sodium zirconate, which is then subjected to acidification, precipitation, and calcination to obtain zirconia. This method yields high purity but is complex and costly.
Electromelting Method: Zircon is melted at high temperatures in an electric arc furnace, and reducing agents such as carbon are added to promote the reaction, causing the zircon to decompose into zirconia and silicon dioxide. The silicon dioxide escapes in gaseous form, thus enriching the zirconia. This method is less expensive and suitable for large-scale production. - Stabilizer Addition: To improve the performance of zirconia, stabilizers such as yttrium oxide (Y₂O₃) and calcium oxide (CaO) are often added to form stabilized or partially stabilized zirconia. This inhibits phase transitions during high-temperature cooling, improving the material’s toughness and stability.
- Dry Pressing: Zirconia powder is mixed uniformly with an appropriate amount of binder and lubricant, then placed in a mold and compressed using a press to compact and shape the powder. This method is simple and suitable for products with simple shapes and small sizes, but the uniformity of the green body density is relatively poor.
- Isostatic Pressing: The powder is placed in an elastic mold and then into a high-pressure container. Pressure is applied uniformly through a liquid medium, causing the powder to be compressed and shaped simultaneously in all directions. This method yields green bodies with uniform density and high strength, and is suitable for products with complex shapes and high performance requirements.
- Slip Casting: Zirconia powder is mixed with water, dispersants, etc., to form a slurry, which is then poured into a porous gypsum mold. The gypsum mold’s water absorption gradually removes the water from the slurry, and the powder particles deposit on the mold wall to form the shape. This method is suitable for manufacturing large, complex-shaped ceramic components, but the green body density and strength are lower.
- Hot Press Casting: At a higher temperature (60-100درجة مئوية), zirconia powder is mixed with binders such as paraffin wax to form a slurry. The slurry is injected into a metal mold using compressed air. After holding pressure and cooling, the wax mold is removed, and then dewaxed to obtain the green body. This method produces green bodies with precise dimensions and high production efficiency, but it is not suitable for manufacturing large components.
- Tape Casting: Zirconia powder is thoroughly mixed with organic binders and plasticizers to form a viscous slurry. The slurry is uniformly coated onto a conveyor belt using a doctor blade to control the thickness. After drying, a thin film green body is obtained. This method is suitable for preparing thin film materials, but requires precise control of process parameters.
- Injection Molding: Zirconia powder is mixed with a thermoplastic binder to form an injection molding material. This material is then injected into a mold using an injection molding machine. This method can produce products with complex shapes and high precision, and has high production efficiency, but requires high-quality raw materials and equipment.
- Pressureless Sintering: The formed green body is placed in a high-temperature furnace and heated to a certain temperature (usually 1300-1500℃) in an atmospheric environment, allowing the particles in the green body to diffuse and bond with each other, achieving densification. This is the most common sintering method, simple to operate, but requires high sintering temperatures and consumes a lot of energy.
Hot Pressing Sintering: Pressure and temperature are applied simultaneously during the sintering process, allowing the green body to densify more quickly under pressure. This method can lower the sintering temperature and improve the density and performance of the green body, but the equipment is complex and the cost is high. - Hot Isostatic Pressing (HIP): The green body is placed in a high-pressure vessel, and high temperature and high pressure are applied simultaneously, allowing the green body to densify under uniformly distributed pressure. This method can obtain high-density, high-performance ceramic materials, but the equipment is expensive and the production cost is high.
- Microwave Sintering: This method utilizes the interaction between the microwave electromagnetic field and the ceramic material to generate heat inside the material, achieving rapid sintering. This method has the advantages of uniform heating, short sintering time, and low energy consumption, but it requires high demands on raw materials and process parameters.
- Spark Plasma Sintering (SPS): A pulsed current is used to generate a discharge plasma between powder particles, producing high temperature and high pressure, allowing the green body to densify rapidly. This method has low sintering temperature and short sintering time, and can obtain high-density, fine-grained ceramic materials, but the equipment is complex and the cost is high.
- After sintering, zirconia ceramics may require further processing and treatment, such as grinding, polishing, cutting, and drilling, to meet the dimensional accuracy and surface quality requirements of the product. In addition, for some special applications, surface coating and modification treatments may be necessary to improve the performance and functionality of the ceramic.
Applications of zirconia ceramics
- Dental Restorations: Used for making dental crowns, bridges, inlays, and implants, offering excellent biocompatibility, aesthetics, high strength, and wear resistance. They effectively replace damaged teeth, restoring chewing function and appearance.
- Artificial Joints: Such as artificial hip and knee joints, utilizing their high strength, wear resistance, and biocompatibility to reduce the body’s rejection of implants and extend their lifespan.
- Surgical Instruments: Some surgical instruments use zirconia ceramic material, which is corrosion-resistant, rust-proof, and maintains its sharpness for a long time, making them suitable for surgical procedures requiring high hygiene and precision.
- Integrated Circuit Substrates: As a high-performance insulating material, it is used in the packaging and substrates of integrated circuits, possessing excellent electrical insulation, thermal stability, and mechanical strength, which helps to improve the performance and reliability of electronic devices.
- High-Frequency Insulating Materials: In high-frequency electronic equipment such as 5G communication and radar, zirconia ceramics can be used as an insulating layer or dielectric material, meeting the needs of high-frequency signal transmission and reducing signal loss and interference.
- Piezoelectric Ceramics: Utilizing its piezoelectric effect, it is used to manufacture sensors, transducers, and actuators, such as pressure sensors, acceleration sensors, and ultrasonic transducers. It is widely used in fields such as automation control, medical diagnostics, and environmental monitoring.
- Engine components: such as turbine blades, combustion chamber liners, and nozzles, utilize their high-temperature resistance, high strength, and low density to improve engine efficiency and reliability, and reduce aircraft weight.
- Thermal protection systems: used as thermal tiles and thermal protection coatings, they protect the aircraft from erosion by high-temperature airflow during high-speed flight or atmospheric re-entry, ensuring the safety of the aircraft structure.
- Satellite antenna supports: utilizing their lightweight, high rigidity, and dimensional stability, they are used to manufacture support structures for satellite antennas, improving the pointing accuracy and stability of the antennas.
- Engine components: Such as engine cylinder liners, piston crowns, and valve seat rings, utilize the excellent thermal insulation and wear resistance of zirconia ceramics to improve engine fuel efficiency and power performance, and extend engine service life.
- Oxygen sensors: Used to monitor the oxygen content in engine exhaust, enabling precise control of fuel injection volume, improving combustion efficiency, and reducing exhaust emissions.
- Braking system components: The brake discs and brake pads of some high-end cars use zirconia ceramic materials, which have higher wear resistance, heat resistance, and braking performance, shortening braking distance and improving driving safety.
- Ceramic bearings: Featuring wear resistance, corrosion resistance, high temperature resistance, and self-lubrication without oil, they are suitable for high-speed, high-precision mechanical transmission systems in harsh environments, such as miniature cooling fans, precision instruments, and machine tool spindles.
- Ceramic valves: Used in chemical, petroleum, and metallurgical industries to control fluid flow and pressure, they offer advantages such as corrosion resistance, wear resistance, and high temperature resistance, and can replace traditional metal valves, extending service life.
- Cutting tools: Such as ceramic cutting tools, drills, and milling cutters, they possess high hardness, high strength, and wear resistance, making them suitable for processing high-hardness materials such as cast iron, hardened steel, and high-temperature alloys, improving processing efficiency and accuracy.
- Solid Oxide Fuel Cells (SOFCs): Utilizing their oxygen ion conductivity as an electrolyte material, SOFCs enable efficient fuel conversion and power generation, offering advantages such as high energy conversion efficiency, broad fuel adaptability, and minimal environmental pollution.
- Solid-State Battery Separators: Used in solid-state batteries as an ion conduction channel and electrode isolation layer, these separators possess excellent chemical stability, mechanical strength, and ion conductivity, improving the energy density and safety of the battery.
- Solar Thermal Utilization: As a heat-absorbing or insulating material in solar collectors, its high-temperature resistance, corrosion resistance, and good thermal conductivity improve the efficiency of solar energy utilization.
- Fiber optic ferrules and sleeves: Used in fiber optic connectors, these components feature high precision, low insertion loss, and high stability, ensuring efficient transmission of fiber optic signals and reliable connections. They are critical components in optical communication networks.
- Optical isolators and circulators: Utilizing the optical and mechanical properties of zirconia ceramics, these components are manufactured to create optical isolators and circulators, enabling unidirectional transmission and routing of optical signals, thereby improving the performance and stability of optical communication systems.
Frequently Asked Questions About Alumina Ceramics
What are the main advantages of zirconia ceramic?
Zirconia ceramic is valued for its high strength and fracture toughness.
Compared with most technical ceramics, it is more resistant to cracking and impact.
This makes it suitable for applications where mechanical reliability is critical.
Is zirconia ceramic stronger than alumina ceramic?
Zirconia ceramic generally has higher fracture toughness than alumina ceramic, which means it is less brittle under mechanical stress.
الألومينا, however, may offer better wear resistance and thermal stability in certain conditions.
The best choice depends on how the component fails in real operation.
What is yttria-stabilized zirconia (YSZ)?
Yttria-stabilized zirconia is zirconium oxide combined with yttrium oxide.
This stabilization improves phase stability and toughness.
It allows the material to maintain strength under load and thermal stress.
Can zirconia ceramic be used in high-temperature environments?
Zirconia ceramic performs well at elevated temperatures and has low thermal conductivity.
It is often used where heat insulation or thermal stability is required.
For long-term exposure or rapid thermal cycling, operating limits should be carefully evaluated.
Is zirconia ceramic suitable for wear or sliding applications?
Zirconia ceramic can be used in wear applications, especially where impact resistance is important.
For pure abrasive wear conditions, other ceramics such as alumina or silicon carbide
may offer better performance depending on system design.
How does zirconia ceramic perform in corrosive environments?
Zirconia ceramic shows excellent resistance to most acids, alkalis, and industrial chemicals.
This makes it suitable for chemical processing and corrosive operating environments
where metal components may fail quickly.
Can zirconia ceramic components be customized?
Yes. Zirconia ceramic components can be customized in material grade, size, shape,
surface finish, and tolerance.
Customization helps ensure proper fit and stable performance under specific operating conditions.
 جيفينج سيراميك
جيفينج سيراميك
